Liming Soils: Good Information to Remember

I had the pleasure of teaming up with Dr. Tom Samples many years ago at a Tennessee Turfgrass Conference. Together we discussed soil chemistry and nutrient interaction and topdressing choices. I recall it was a great seminar and the room was full. At one point a representative from the Grand Ole Opry Hotel came in and said, “no more people could come in” due to fire safety rules.
The following article was written by Dr. Samples, Ph.D., John Sorochan, Ph.D., Jim Brosnan, Ph.D, Hugh Savoy – Bio systems Engineering and Soil Science Dept and Alan Windham, Ph.D from the University of Tennessee.
So you have read our articles and heard us speak about soils and balance of nutrients and the importance of calcium! No you can read what they have to say. Although much of this information is repetitive, it drives home a strong point! Soil Balance is Important.
Read on…
The Importance of Liming Acidic Soils
By Tom Samples, Ph.D., John Sorochan, Ph.D., and Jim Brosnan, Ph.D., Plant Sciences Dept.; Hugh Savoy, Biosystems Engineering and Soil Science Dept.; and Alan Windham, Ph.D., Entomology and Plant Pathology Dept., The University of Tennessee
Unless lime is applied, many Tennessee soils in which turfgrasses are maintained become acidic. Soil acidity increases as turfgrasses remove calcium (Ca), magnesium (Mg) and potassium (K), or as the nutrients move in water (leach) below the turfgrass rootzone. Nitrogen (N) fertilization, and collecting and removing grass clippings, can also result in acidic soils. As the total amount of acids in soils increases, several essential nutrients change form and are no longer available to turfgrass plants. The activity of many species of beneficial microorganisms in soils may also slow.
Soil pH
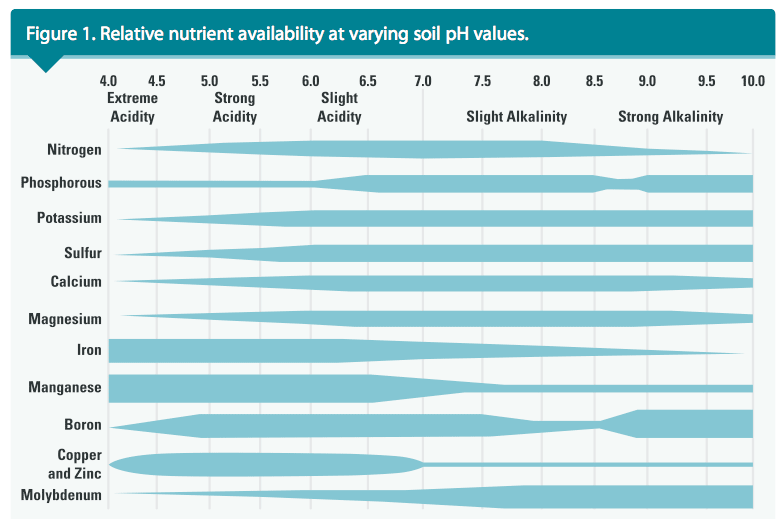
The term pH is a notation used to designate the level of acidity or alkalinity of a soil, where p represents “potential,” and H represents hydrogen. When subjected to a soil pH test, strongly acidic soils yield very high levels of H or aluminum (Al) ions. Soil with a pH below 7.0 is acidic, and above 7.0 is alkaline. Soil with a pH of 5.0 is 10X more acidic than the same soil with a pH of 6.0 and 100X more acidic than the soil with a neutral pH (pH = 7.0).
The amount of lime needed to neutralize the acids in a soil depends on the soil’s pH and the ability of the soil to resist (buffer) a change in pH. Clayey soils have a greater buffering capacity than sandy soils.
Availability of essential mineral nutrients
In native soils, the essential mineral nutrients are available for uptake by turfgrasses when the soil pH is slightly acidic (ranging from 6.0 to 6.5). Soil with a pH of 5.5 or less may be very low or deficient in Ca, Mg and phosphorus (P), yet may contain excessive Al and manganese. See Figure 1 for more information about nutrient availability in soils of varying pH.
Turfgrass weeds and diseases as indicators of soil pH
The presence of several broadleaf weeds including cinquefoil (Potentilla simplex), ground ivy (Puccinia glechomatis), sheep sorrel (Rumex acetosella) and Shepherds purse (Capsella bursa-pastoris) may indicate low soil pH and a high level of soil acidity. Conversely, the presence of common plantain (Plantago major) may indicate that the soil is alkaline and has a relatively high pH. Similarly, the fungal diseases Fusarium patch, spring dead spot, summer patch and take-all patch can be much more severe when turfgrasses are growing in alkaline soils.
Soil pH testing
Soil testing is a key to determining when it is necessary to apply lime and the amount of lime to apply. A glass electrode is used to estimate the pH of the soil sample De-ionized water is added to dry, pulverized soil before measuring the level of acid in the soil sample solution. The pH of this solution is often reported on the soil test result form as Water pH. Water pH is used to indicate a need for lime. The amount of acid held (adsorbed) by clay and organic matter in the soil sample that must be neutralized is often reported as Buffer pH.
Liming materials
Agricultural or ground limestone is the main source of lime for turf applications. Agricultural lime is marketed in either calcitic or dolomitic forms and is sold in bulk or bags. The most common form of bagged limestone is dolomitic. Calcitic limestone contains mostly Ca carbonate (CaCO3), while dolomitic limestone contains both Ca plus Mg carbonates (CaMg(CO3)2).
Particles of ground limestone are often compressed to form larger granules or pellets. Although pelletized lime is usually much easier to apply, if the lime pellets do not break down quickly once they contact rain or irrigation water, their effectiveness in raising the soil pH may be reduced. Pelletized lime may require more time to raise the soil pH of the turfgrass rootzone than ground limestone.
Role of calcium and magnesium in turfgrasses
As part of the cell walls and membranes, Ca contributes to overall plant strength. Calcium is also required in order for cells to divide and expand. The amount of Ca2+ in the soil solution surrounding turfgrass roots influences the amount of K and sodium (Na) cations a turfgrass plant absorbs. For example, when the solution is low in Ca2+, the amount of K+ and Na+ absorbed is often very similar. However, when the supply of Ca2+ in soil solution is adequate, much more K+ is absorbed than Na+. Calcium also improves the uptake of nitrate N (NO3-).
Magnesium is part of the chlorophyll molecule and is critically important for photosynthesis. The plant’s ability to produce proteins and transfer energy from one plant part to another is affected by Mg. An adequate level of Mg also improves the uptake of P from soil.
Application timing
Although late fall is preferred, lime can be applied to turf any time during the year. Rotary (centrifugal) spreaders are commonly used to apply pelletized lime, while pulverized lime should be applied using a drop- or gravity-spreader.
When establishing turfs, the total recommended amount of lime can be broadcast and tilled into the soil before planting. In established turfs, no more than 50 lbs. of lime should be applied per 1,000 ft2. For example, if soil test results indicate the need to apply a total of 150 lbs. of lime per 1,000 ft2, 50 lbs. per 1,000 ft2 can be applied on 6-month intervals such that 50 lbs. per 1,000 ft2 is applied initially, an additional 50 lbs. per 1,000 ft2 is applied after 6 months, and the final 50 lbs. per 1,000 ft2 application is made 1 year after the initial application.
Liming acidic soils most often results in healthier turfgrass and reduces the need to supplement those mineral nutrients that were once unavailable to turfgrasses.